Three scientists have been awarded the 2025 Nobel prize in chemistry for discovering a new form of molecular architecture: crystals that contain large cavities.
Susumu Kitagawa from Kyoto University, Japan, Richard Robson from the University of Melbourne, Australia, and Omar M. Yaghi from the University of California, Berkeley, in the US, will share a prize sum of 11 million Swedish kronor (£870,000).
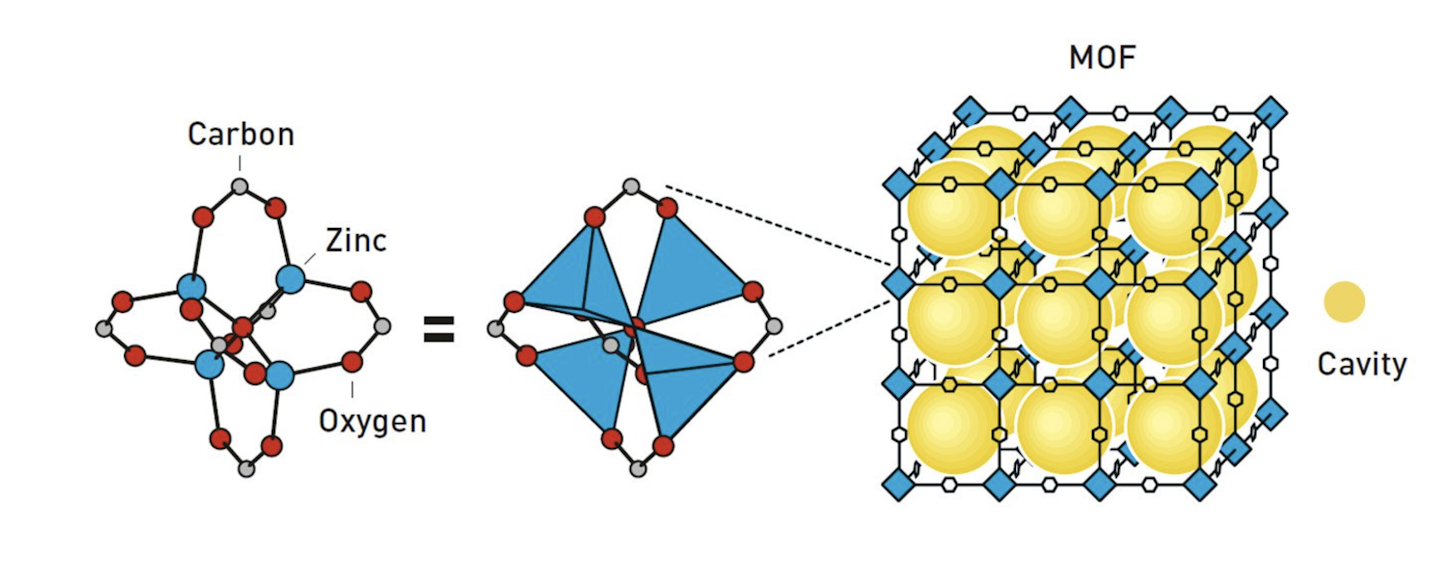
The prize recognises the pioneering contributions of the three scientists in the development of something called metal-organic frameworks (Mofs). Mofs are a diverse class of crystalline materials that have attracted much attention in chemistry due to the presence of microscopic open cavities in their structures. They are helping to revolutionise green technology, such as harvesting water from desert air and capturing CO₂.
The widths of the cavities can range from a few angstroms (an angstrom is a unit of length equal to one hundred-millionth of a centimetre) to several nanometres (a millionth of a millimetre). That means they are far too small to see with the naked eye or even with most forms of microscopes. But they’re the perfect size for housing various molecules.
The development of Mofs can be traced back to the late 1950s when researchers started to discover “coordination polymers”. These are materials made up of linked chains of metal ions (atoms that have lost or gained electrons) and carbon-based bridging molecules known as linkers. These materials did not contain cavities, but they were based on the same metal-organic chemistry that would later give rise to Mofs.
In the late 1980s, Robson’s research group reported that some coordination polymers could be prepared as framework-like structures where, crucially, the carbon-based linkers formed three-dimensional arrangements around clusters of liquid solvent molecules. As mentioned in Robson’s research article, this revealed “an unusual situation in which approximately two-thirds of the contents of what is undoubtedly a crystal are effectively liquid”.
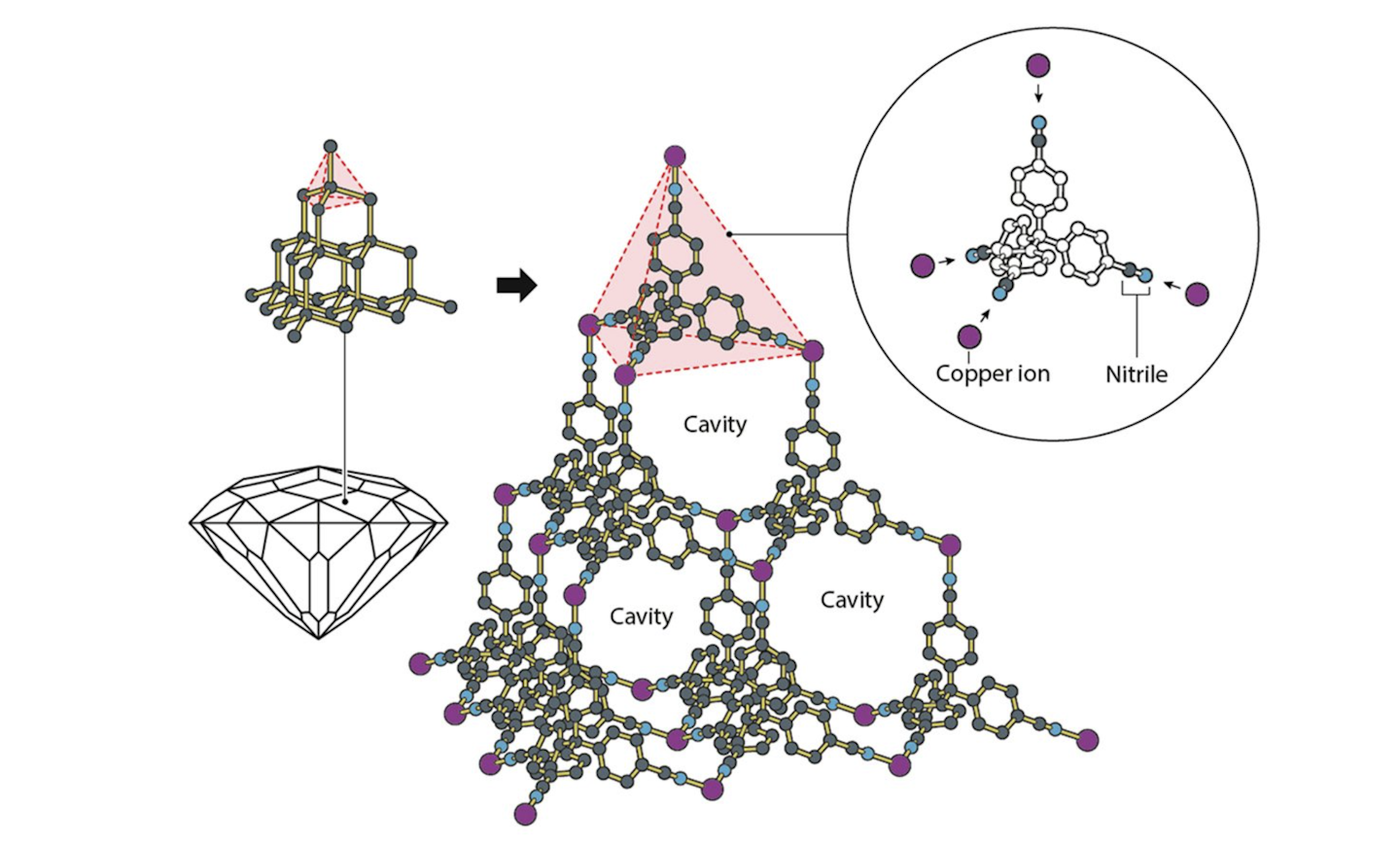
In the mid-late 1990s, Yaghi’s group demonstrated that it was possible to prepare coordination polymers that retained their structures even after the solvent molecules were removed from the cavities. This was a surprising result, which dispelled the prevailing assumption that such frameworks are fragile and would collapse if the solvent was removed.
In 1997, Kitigawa’s research group showed that the open cavities could be used to absorb gas molecules. He also showed that, in many cases, the framework itself expands as gas molecules are absorbed into it and contracts as they are released. These coordination polymers with permanent, open cavities came to be known as Mofs.
The discoveries by the three scientists effectively marked the birth of modern Mof chemistry, with many thousands of research articles published on them since.
Wide range of applications
Why are Mofs so interesting for chemists? The microscopic cavities within Mofs provide a unique and controllable location for chemistry to take place. A key application of Mofs is gas storage. In many cases, these materials can hold gases at much higher densities than in their free gaseous state.
This offers significant advantages for green technologies such as fuel-cell-powered vehicles, in which hydrogen fuel has to be transported as efficiently as possible. Many Mofs work particularly well for specific gases, which means they can also help separate gas mixtures in exhaust streams, or capture CO₂ from the air to mitigate the effects of global warming.
Mofs can also act as effective catalysts for chemical reactions taking place in the cavities. One of the key advantages of Mofs as catalysts is that it is relatively straightforward for chemists to switch and swap the metals and carbon-based linkers in order to tune the properties for a particular purpose.
As well as gas molecules, Mofs can also accommodate other small molecules, such as pharmaceuticals. This means they can be used to store and deliver drugs to a particular target, where their porous nature allows for controlled release of therapeutic chemicals.
In recent years, Mofs have shown promise for many other applications, including batteries, thermal energy storage and chemical sensors (devices that can monitor and detect chemicals such as contaminants). Excitingly, there remain many other applications that have yet to be explored.
Despite having been discovered over three decades ago, Mofs remain one of the hottest research areas in materials chemistry and will no doubt do so for many years to come.
This article is republished from The Conversation, a nonprofit, independent news organization bringing you facts and trustworthy analysis to help you make sense of our complex world. It was written by: John Griffin, Lancaster University
Read more:
- Nobel physics prize awarded for pioneering experiments that paved the way for quantum computers
- Nobel prize awarded for discovery of immune system’s ‘security guards’
- Nobel medicine prize: how a hidden army in your body keeps you alive – and could help treat cancer
John Griffin receives funding from the Engineering and Physical Sciences Research Council (EPSRC) and the Faraday Institution, and has previously received funding from the Leverhulme Trust.


 The Conversation
The Conversation
 Local News in Wisconsin
Local News in Wisconsin People Top Story
People Top Story AlterNet
AlterNet Hattiesburg American
Hattiesburg American CBS Philly
CBS Philly Breitbart News
Breitbart News