After a routine C-section at an Auckland hospital, a mother developed severe pain and what seemed like postnatal fatigue. It turned out to be an infection with methicillin-resistant Staphylococcus aureus (MRSA), a “superbug” spreading across New Zealand and beyond.
Her story isn’t unique. In Dunedin, another mother was diagnosed with MRSA and internal bleeding postpartum.
These are personal stories, but they are also early warning signals of a broader health security challenge.
Methicillin is an antibiotic from the penicillin family, and MRSA is resistant to it (and often to other types of antibiotics). This makes infections harder to treat.
Once considered mainly a hospital problem, methicillin-resistant infections are now common in the community.
An MRSA strain that emerged in New Zealand (named AK3) is now the dominant strain in our communities. The country’s antibiotic-dispensing habits may have helped it emerge and spread.
A New Zealand-born strain with global reach
AK3 was first detected in 2005. Today, it is the leading cause of MRSA infections in New Zealand and, as our new study shows, has also been detected in the South Pacific and Europe.
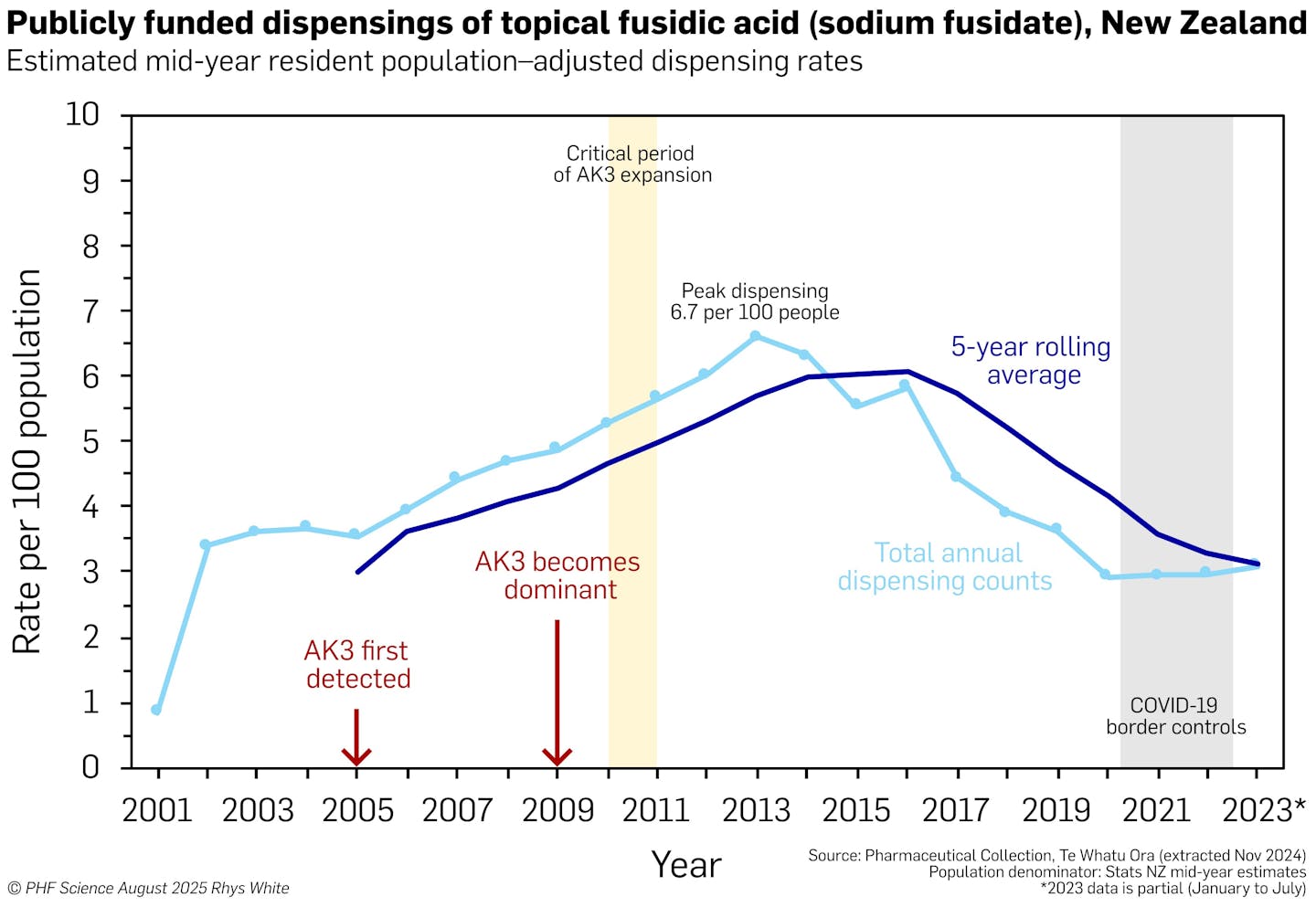
Using whole-genome sequencing, we traced the journey of AK3 from a drug-susceptible ancestor to a highly resistant clone. Along the way, AK3 acquired key resistance genes, including those conferring resistance to methicillin and fusidic acid, a topical antibiotic once prescribed at historically high rates in New Zealand.
Dispensing of topical fusidic acid has declined since 2016. But once resistance becomes common, it can be difficult to reduce or revert to a susceptible form. This is why it is so important to preserve the effectiveness of antibiotics by using them carefully and only when needed (a practice known as antimicrobial stewardship), and to monitor resistance trends using genomic surveillance.
How a common prescription helped a superbug thrive
In New Zealand, fusidic acid was once widely prescribed in the community to treat skin infections, often without a confirmed diagnosis. This widespread use created ideal conditions for fusidic acid resistance to develop.
The emergence of AK3 demonstrates how antibiotics, when overused at a national scale, can drive the evolution of globally significant resistance. Our genomic study has improved our understanding of how AK3 evolved and became dominant in New Zealand.
Our analyses and public health data show that AK3 does not affect all groups equally. Compared with people of European ethnicity, Māori are three times more likely, and Pacific peoples nearly five times more likely, to suffer from skin infections caused by S. aureus.
Socio-economic factors amplify the risk: those in the most deprived areas are nearly four times more likely to be hospitalised with skin infections.
The burden of skin infections and overuse of fusidic acid created the conditions for AK3 to emerge, adapt and spread. For people with AK3 and other resistant superbugs, it is critical to preserve the effectiveness of antibiotics. Fusidic acid still has a role in treatment, but the goal is to strike the right balance between under- and over-prescribing.
Addressing this requires improved access to timely, appropriate care and treatment in the communities most affected. This is a reminder that even the most advanced technologies cannot, on their own, overcome structural barriers. If we are serious about the threat antimicrobial resistance poses, we must confront these inequities head-on.
What next?
Recently, we detected AK3 in a sample of raw milk collected directly from a cow with mastitis during veterinary testing – well before any processing, and not from milk intended for people to drink. Resistance genes can cross human, animal and environmental boundaries and this demands a “One Health” approach, integrating surveillance with coordinated policy and action across all domains.
Surveillance is the continuous and systematic collection of data to inform public health action, typically drawing from multiple sources, including laboratory results, epidemiological information and genomic analysis.
The rise of AK3 underscores the need for a proactive, integrated antimicrobial resistance strategy across science, policy, veterinary and public health. Genomics is a key strategic priority, supporting an evidence-based approach to current and emerging diseases.
The case of AK3 brings these priorities into sharp focus and shows why coordinated action is essential to stay ahead of emerging threats.
To protect New Zealand’s health security and economy, we must support appropriate antimicrobial use. The right antibiotic should be used at the right time and in the right dose, guided by local data on microbial resistance and supported by accessible diagnostics.
We must also expand antimicrobial resistance surveillance and strengthen monitoring across human, animal and environmental health, with genomic analysis as a core component so that threats can be detected and transmission disrupted earlier.
The stories of two mothers whose MRSA infections complicated childbirth are more than case studies. They are calls to action and AK3 is our test. It challenges us to move from reactive treatment to proactive prevention.
New Zealand has the tools, talent, technology and networks to lead in antimicrobial surveillance and response. But we must act decisively. Not just to contain AK3, but to prevent the next superbug from emerging.
Let’s treat antibiotics like the critical infrastructure they are, recognising that the pipeline for new antibiotics is very narrow. We must make sure antibiotics will still work when we need them most.
We acknowledge the contributions by Max Bloomfield and the teams at Awanui Labs, Emma Voss and team at Livestock Improvement Corporation and all collaborators, including from New Zealand’s diagnostic labs, Health New Zealand, Ministry of Health, Anexa Veterinary Services, University of Auckland, and University of Otago.
This article is republished from The Conversation, a nonprofit, independent news organization bringing you facts and trustworthy analysis to help you make sense of our complex world. It was written by: Rhys Thomas White, New Zealand Institute for Public Health and Forensic Science; Juliet Elvy, New Zealand Institute for Public Health and Forensic Science; Kristin Dyet, New Zealand Institute for Public Health and Forensic Science; Maria Hepi, New Zealand Institute for Public Health and Forensic Science; Nigel French, Te Kunenga ki Pūrehuroa – Massey University, and Sarah Bakker, New Zealand Institute for Public Health and Forensic Science
Read more:
- Do you really need antibiotics? Curbing our use helps fight drug-resistant bacteria
- No, antibiotics aren’t always needed. Here’s how GPs can avoid overprescribing
- How do bacteria actually become resistant to antibiotics?
Rhys White received a travel bursary from Oxford Nanopore Technologies and a travel grant from the UK Microbiology Society. This study was supported by internal departmental funds at Awanui Laboratories Wellington, the New Zealand Institute for Public Health and Forensic Science (PHF Science), and Genomics Aotearoa.
Kristin Dyet receives funding from the New Zealand Public Health Agency.
Maria Hepi receives funding from Te Niwha Infectious Disease Platform
Nigel French receives funding from the NZ Ministry of Business Innovation and Employment, the Health Research Council and Te Niwha.
Juliet Elvy and Sarah Bakker do not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.


 The Conversation
The Conversation
 Newsweek Top
Newsweek Top Associated Press Top News
Associated Press Top News NBC Southern California
NBC Southern California Raw Story
Raw Story KNAU
KNAU RadarOnline
RadarOnline NBC Bay Area Dixon News
NBC Bay Area Dixon News People Top Story
People Top Story Daily Kos
Daily Kos