Most people have heard of stem cells. They are often described as “miracle” cells –ones that can grow into any other type of cell in our bodies, promising revolutionary medical treatments.
However, not all stem cells are the same, and not all of them can become treatments equally well.
To appreciate what stem cells actually do – and can’t do – we need to understand their different types. Each comes with its own strengths, limitations, and challenges.
Stem cells already save lives in Australia and worldwide. But if we want them to help more people, science alone is not enough. We also need strong regulation, industry partnerships and public trust.
What are the three types of stem cells?
Stem cells are the body’s raw materials: unspecialised cells that can, under the right conditions, develop into many different types of specialised cells including blood, skin, heart, or brain.
There are three main stem cell types: adult, embryonic, and induced pluripotent.
As their names suggest, they are found in adult tissues, come from embryos, or are created in the lab respectively. Let’s look at each type in detail.
Adult stem cells: proven but limited
Adult stem cells are found throughout the body, often named after the tissue they come from – such as bone marrow, skin, or gut.
Because they are collected from a donor or the patient themselves, their use is ethical and based on informed consent. But they are limited. They can usually only regenerate the cell types from the tissue they came from – a skin stem cell can only grow into a skin cell, for example. Also, their quality varies from person to person.
Adult stem cells are useful and can be life saving, but not a universal solution.
The only approved stem cell therapies currently used in Australia involve blood stem cells (haematopoietic stem cells). These are used in bone marrow transplants to treat blood cancers like leukaemia, and some immune conditions such as multiple sclerosis.
Embryonic stem cells: powerful but controversial
Embryonic stem cells are more versatile than adult ones. They appear only days after fertilisation and can become nearly any cell type in the body, a property called pluripotency.
This power comes with ethical and legal challenges. In Australia, embryonic stem cells can only be derived from donated embryos under strict conditions. Their use is tightly regulated and often debated.
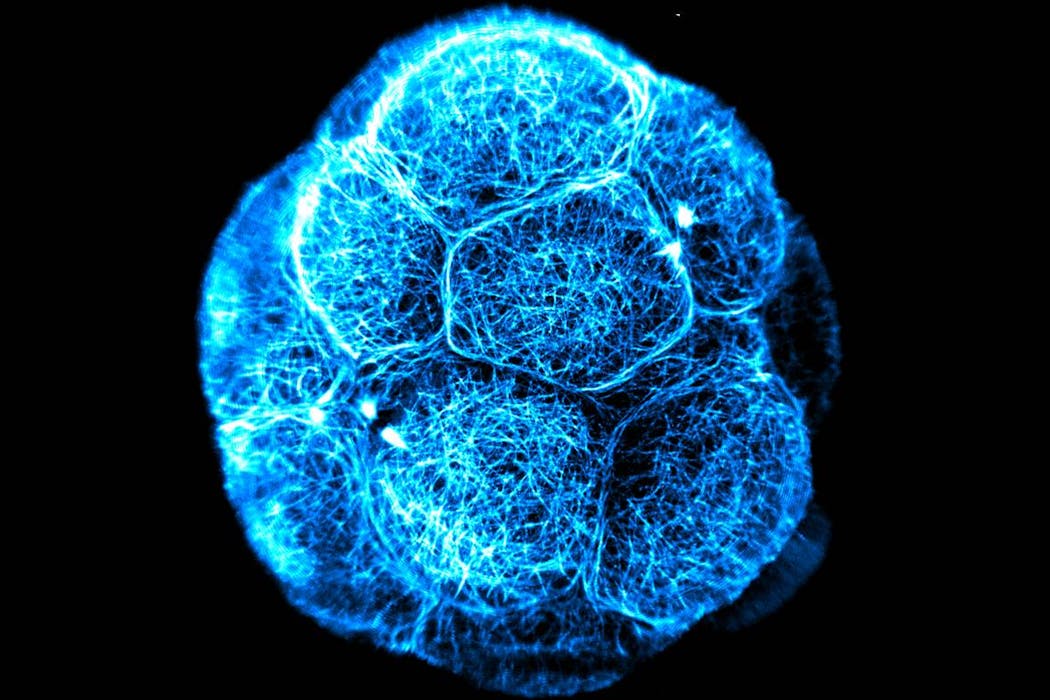
At the Australian Regenerative Medicine Institute, my team studies the earliest stages of life. Using advanced imaging, we capture how embryonic cells organise, change shape, and “decide” what types of cells they will become.
These processes hold vital clues for guiding stem cells to one day repair or replace damaged organs, and for understanding how a healthy embryo develops.
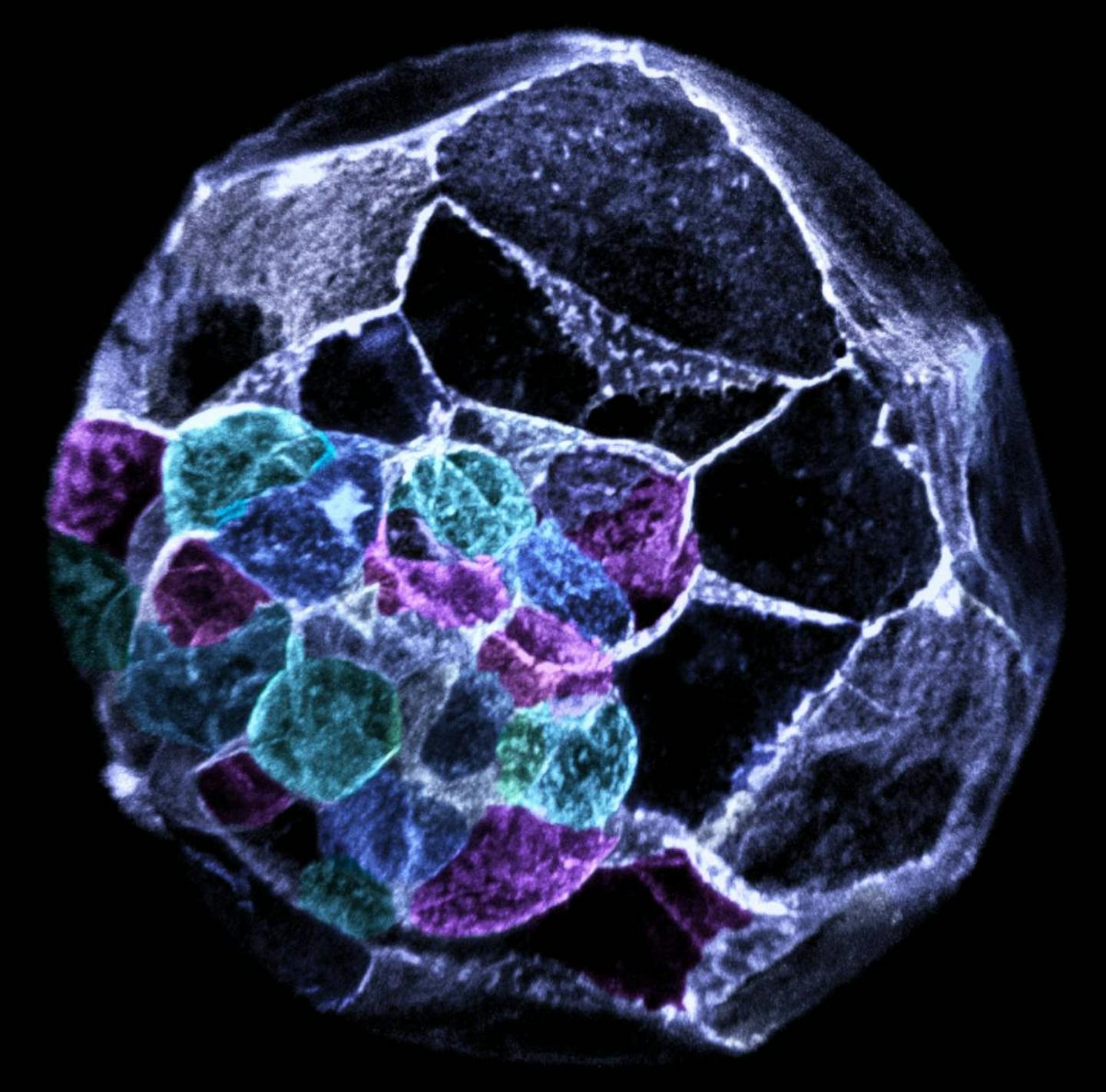
Induced pluripotent stem cells: reprogramming the body
In 2006, scientists found a way to “rewind” specialised adult cells, such as skin or blood cells that usually cannot change, and return them to a stem-cell-like state.
These are called induced pluripotent stem cells or iPSCs for short. Once reprogrammed, they regain the ability to become many other cell types.
iPSCs avoid many ethical issues because they don’t require embryos. They can also be made from a patient’s own cells, lowering the risk of immune rejection.
At our institute, we use this type of stem cells to model diseases, develop new drugs, and generate specialised cells such as neurons, heart muscle and skeletal muscle.
Using the latest advances in science imaging to reveal differences invisible to the naked eye, we are also investigating how closely iPSCs resemble natural embryonic stem cells. Understanding this will help us use them safely and effectively in the future.
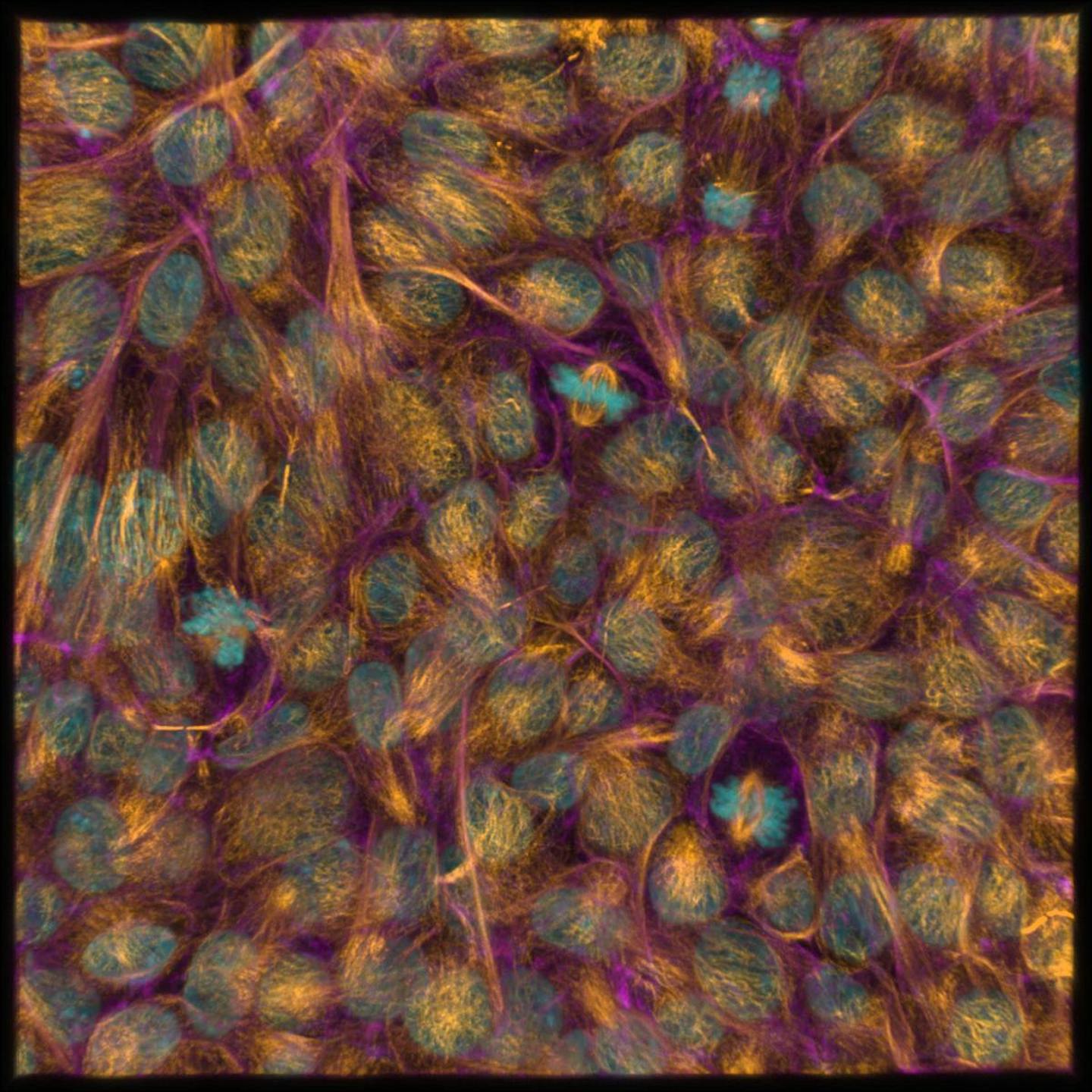
Why aren’t more therapies available yet?
Stem cells have enormous potential, but the path to turning them into proven therapies is complex. Embryonic stem cells and iPSCs face major scientific, technical and regulatory hurdles.
Any therapy must be shown to be safe, effective and reliably manufactured – a process that takes years of testing and clinical trials.
There is also a risk from unproven stem cell clinics, which offer treatments that lack evidence and can put patients at risk. This is why strong national and international regulation is so important.
Equally important is helping everyone understand stem cells so patients can make safe, informed choices. There’s a careful pathway from discovery to treatment, and it’s important to understand the difference between hope and hype.
Stem cells are sort-of magic, but only when truly mastered. They remain one of the most promising frontiers in modern medicine. Beyond cells alone, researchers are now combining stem cell biology with tissue engineering, 3D modelling of organs (organoids) and embryos, and gene editing to push the boundaries of regenerative medicine which harnesses the body’s regenerative capabilities to repair damaged and diseased tissues.
This article is republished from The Conversation, a nonprofit, independent news organization bringing you facts and trustworthy analysis to help you make sense of our complex world. It was written by: Jennifer Zenker, Monash University
Read more:
- From monkey glands to ‘young blood’: the long, strange history of chasing immortality through transplants
- Growing cocktail of medicines in world’s waterways could be fuelling antibiotic resistance
- New student loan limits could change who gets to become a professor, doctor or lawyer
Jennifer Zenker receives funding from NHMRC and Viertel Foundation.


 The Conversation
The Conversation
 Reuters US Domestic
Reuters US Domestic Raw Story
Raw Story The Daily Beast
The Daily Beast Verywell Health
Verywell Health NBC News
NBC News Associated Press US News
Associated Press US News KCRG Iowa
KCRG Iowa FOX 32 Chicago Crime
FOX 32 Chicago Crime US Magazine Entertainment
US Magazine Entertainment